Table of Contents
Electric charge
What is electric charge?
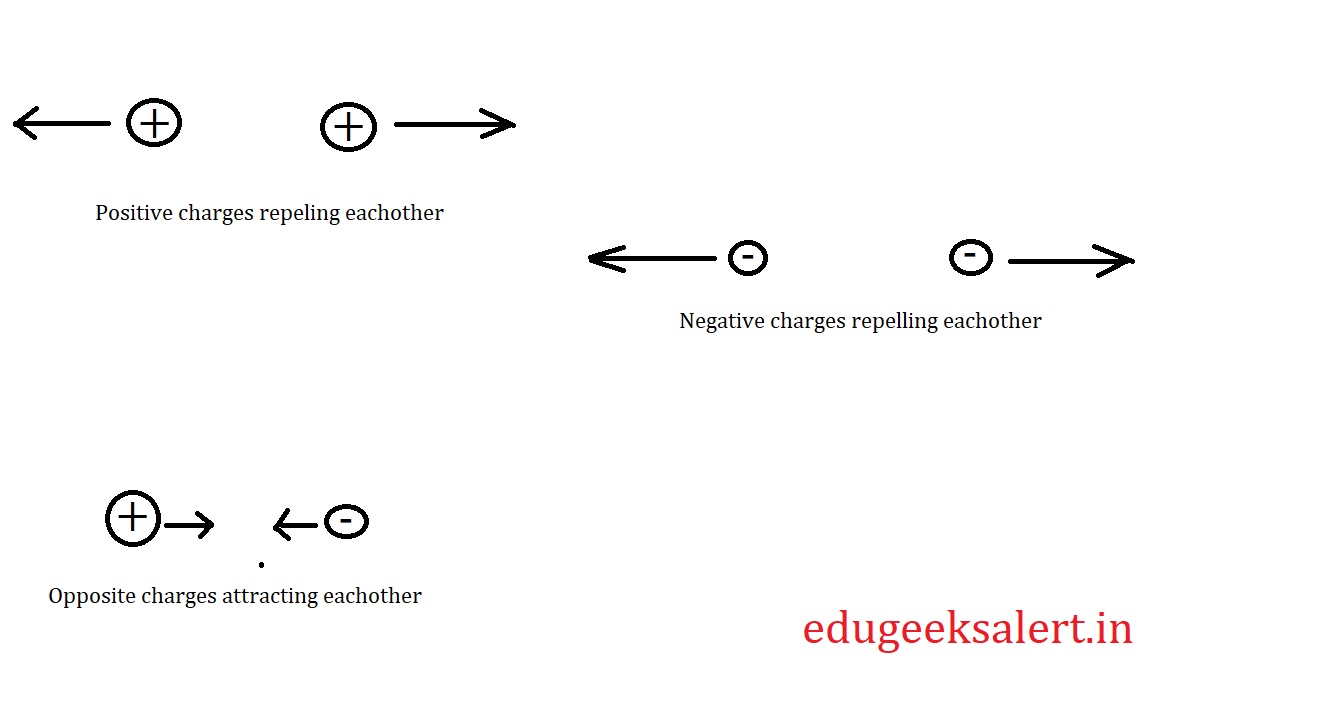
We have three types of particles in nature on the basis of electrostatics. They are namely positive charge particles, negatively charged particles, and neutral particles. They are determined in such a way based on their interaction i.e.
-
like charges repel each other,
-
Unlike charges attract each other, and
-
neutral bodies don’t interact with any other particles.
I.e. Two positive charges always repel each other, and so do the negative charges. Whereas a positive charge always attracts negative charge and vice versa.
Neutral particles are combinations of both positive and negative charges in equal amounts or mathematically we call 0 charge or no charge. As positive attracts negative and negative attracts positive zero being neutral it does not interact with any charge. For example a neutron will not interact with either an electron or a proton.
Informal definition: From the above information we can understand that charge is a physical quantity of particles which determines the nature of electrical interactions.
SI unit of charge is coulomb and represented as C. An electron has a charge of $-1.6 \times 10^{-19}$ C and a proton has a charge of $+1.6 \times 10^{-19}$ C. Whereas a neutron charge is 0 C
*Note: positive and negative is just a representation of the opposite nature of particles in a mathematical way.
Quantization of charge:
Every particle in the universe is made up of atoms, which are made up of electrons, protons and neutrons. Electron is the smallest negative charge while proton is the smallest positive charge there for any charge should be integral multiple of these particles that is a negatively charged particle should be -n e where and is a positive integer and $e = 1.6 \times 10^{-19}$ C. similarly positive charge should be +n e where and is positive integer and $e = 1.6 \times 10^{-19}$. This means that the fractional charge of e doesn’t exist.
Charge is conserved:
A proton and electron can come by to form a Neutron. Similarly a neutron can be divided into a proton and an electron. And in atoms, protons and neutrons makeup the nucleus while electrons revolve around the nucleus in orbits to form a neutral atom.
By this we can understand that charges can be neutralized and charge can be formed from neutral bodies. But the net charge of a closed system is always the same. i.e. net charge of a closed system can neither be destroyed nor created.
Additivity of electric charge:
When two charges combine together the charge of combination is the algebraic sum of the charges of two bodies. i.e. when a 5 C charge body meets with 6 C charge body and stays together then the resultant charge of the new body is 11 coulombs. Similarly when a 5 C charge body meets with -6 C charge body and stays together then the resultant charge of the new body is -1 coulombs.
A real life example of all the above properties of charges can be observed in beta decay. When a neutron in the nucleus of a heavy atom splits into a proton and an electron the charge is conserved because the charge before splitting is zero and the charge after splitting as a whole is 0. Is an example of conservation of charge and an example of additivity of charge.
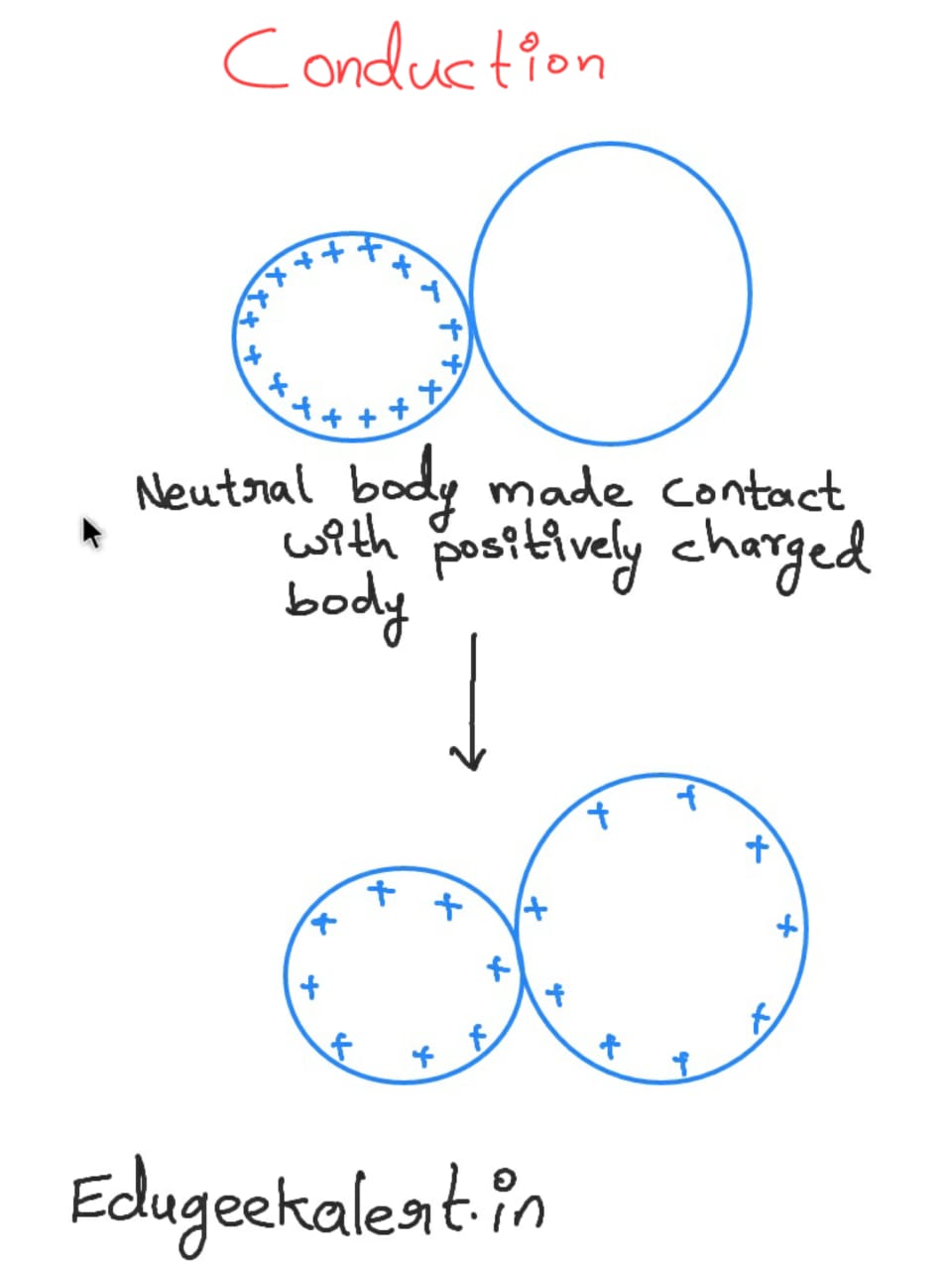
Conduction of charge:
When a charged body is made contact with a neutral conducting body, Charge flows to the neutral body so that charge density is equally distributed in the both bodies. this can be understood by following image.
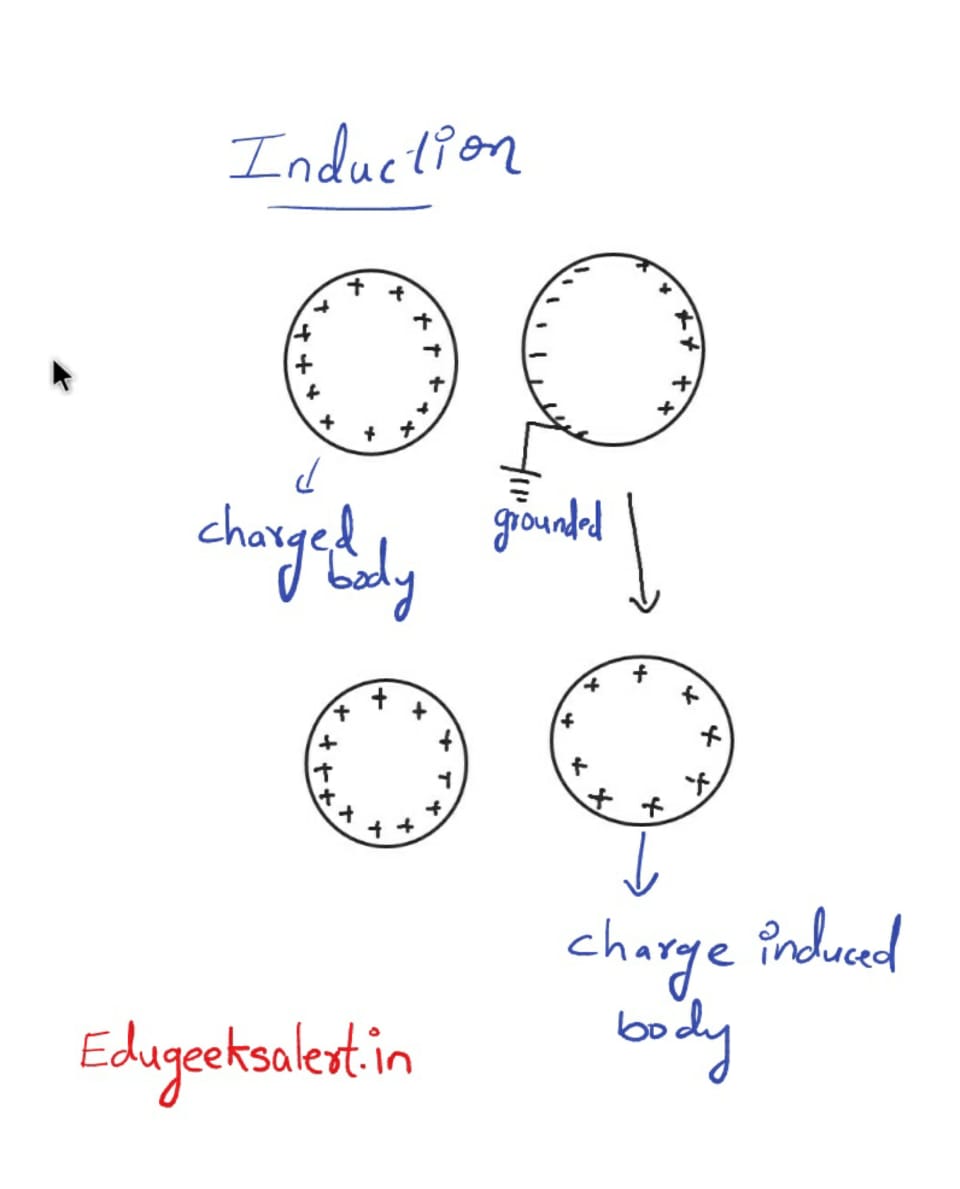
Induction of charge into a neutral body:
Charge can be induced into a body not only by conduction. when a neutral body is brought into the filed of a positively charged body, as shown in the figure, electronss inside the neutral body get attracted towards the positive charge and postive charge get repeled to the otherside as shown in the figure. when we ground the negative charge end, the electrons would floiw to the ground making the body postively charged.
Feedback
Was this page helpful?
Glad to hear it! Please tell us how we can improve.
Sorry to hear that. Please tell us how we can improve.